Evaluation of the Lupus Low Disease Activity State (LLDAS) vs. the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI)-2K Score in a Pediatric Systemic Lupus Erythematosus Cohort
Bridget Wilson, MD; Tingting Qiu, MPH; Angela Merritt; Bin Huang, PhD; Hermine Brunner, MD, MSc, MBA
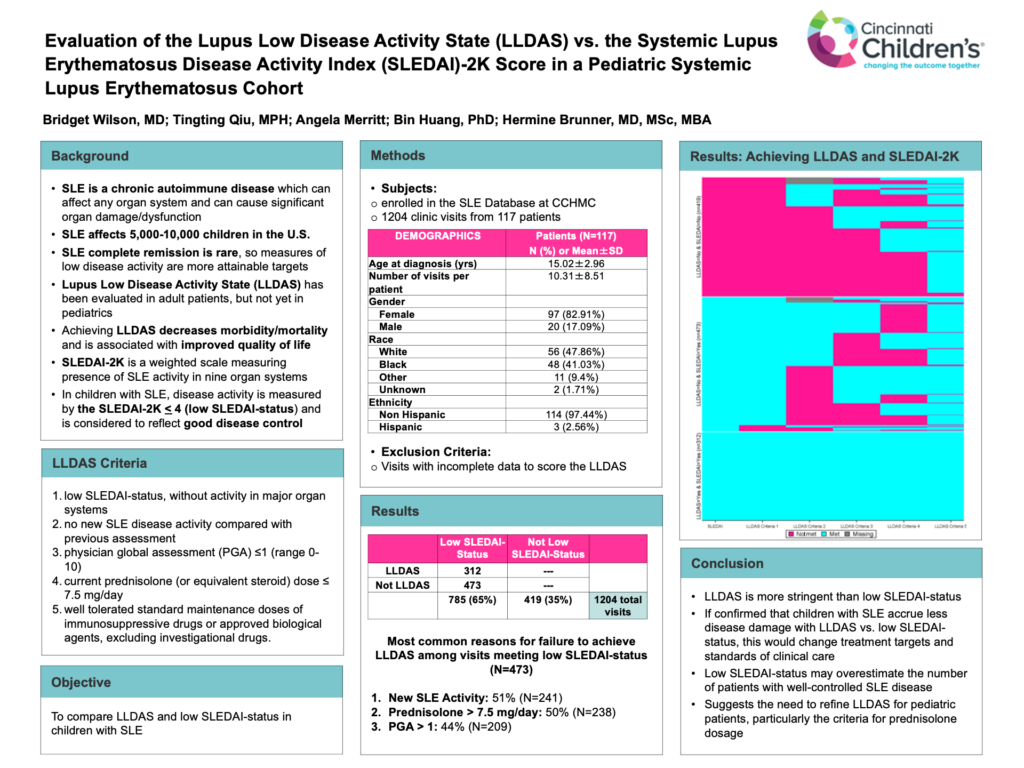
The majority (65%) of SLE clinic visits achieved the current measure of low SLE disease activity, low SLEDAI-status; however, only 40% of these visits also met the more stringent LLDAS criteria.
-Bridget Wilson, MD
Abstract
Background: SLE is a chronic autoimmune disease which can affect any organ system, where ongoing disease activity leads to organ damage. The Lupus Low Disease Activity State (LLDAS) has been evaluated in adult patients, but not yet in pediatrics. In adults, achieving LLDAS was shown to decrease morbidity/mortality and was associated with improved quality of life. The SLEDAI-2K is a weighted scale measuring presence of SLE activity in nine organ systems. In children with SLE, disease activity measured by the SLEDAI-2K < 4 (low SLEDAI-status) is considered to reflect good disease control currently.
Objective: To compare LLDAS and low SLEDAI-status in children with SLE.
Methods: LLDAS metrics were recorded for 1204 clinic visits from 117 patients (83% female; 41% black) enrolled in the SLE Database at CCHMC. Visits with incomplete data to score the LLDAS were omitted. LLDAS criteria include: (1) low SLEDAI- status, without activity in major organ systems; (2) no new SLE disease activity compared with previous assessment; (3) physician global assessment (PGA) ≤1 (range 0-10); (4) current prednisolone (or equivalent) dose ≤ 7.5 mg/day; and (5) well tolerated standard maintenance doses of immunosuppressive drugs or approved biological agents, excluding investigational drugs.
Results: The majority (65%, 785/1204) of visits met low SLEDAI-status criteria, of which only 40% (312/785) met the LLDAS criteria. Of those visits meeting low SLEDAI-status but not meeting LLDAS (N=473), 51% (N=241) presented new SLE activity from the previous visit; 50% (N=238) had current prednisolone (or equivalent) dose >7.5 mg daily, and 44% (N=209) had PGA >1.
Conclusions: LLDAS is more stringent than low SLEDAI-status, which is the current treatment target. If confirmed that children with SLE accrue less disease damage with LLDAS versus low SLEDAI-status, this would change treatment targets, hence the standards of clinical care. The study also suggested the need to refine LLDAS for pediatric patients, particularly the criteria for prednisolone dosage.